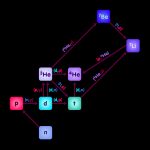
Big Bang Nucleosynthesis: Cooking up the first light elements
How the first nuclei of helium, lithium and other light elements were cooked up shortly after the big bang
An article by Achim Weiss
The big bang models – the cosmological models based on general relativity – tell us that the early universe was extremely hot and dense. At the earliest stages that can be modelled using current physical theories, the universe was filled with radiation and elementary particles – a hot plasma in which energy was distributed evenly. During the subsequent expansion, this plasma has progressively cooled down. By examining how the cooling affects the matter content of the universe, one can derive one of the most impressive testable predictions of the big bang models.
Nuclear physics in an expanding universe
As the universe cools, the matter content changes – new particles are formed out of the preexisting ones, such as protons and neutrons forming out of quarks. From about one second to a few minutes cosmic time, when the temperature has fallen below 10 billion Kelvin, the conditions are just right for protons and neutrons to combine and form certain species of atomic nuclei. This phase is called Big Bang Nucleosynthesis.
While the early universe is totally unlike our everyday world, the basic nuclear physics at the appropriate energies is well within the range of laboratory experiments. Following such experiments, the properties of the relevant nuclear reactions are very well known. Physicists can base their calculations on solid experimental data when they want to describe reactions like the one pictured here:
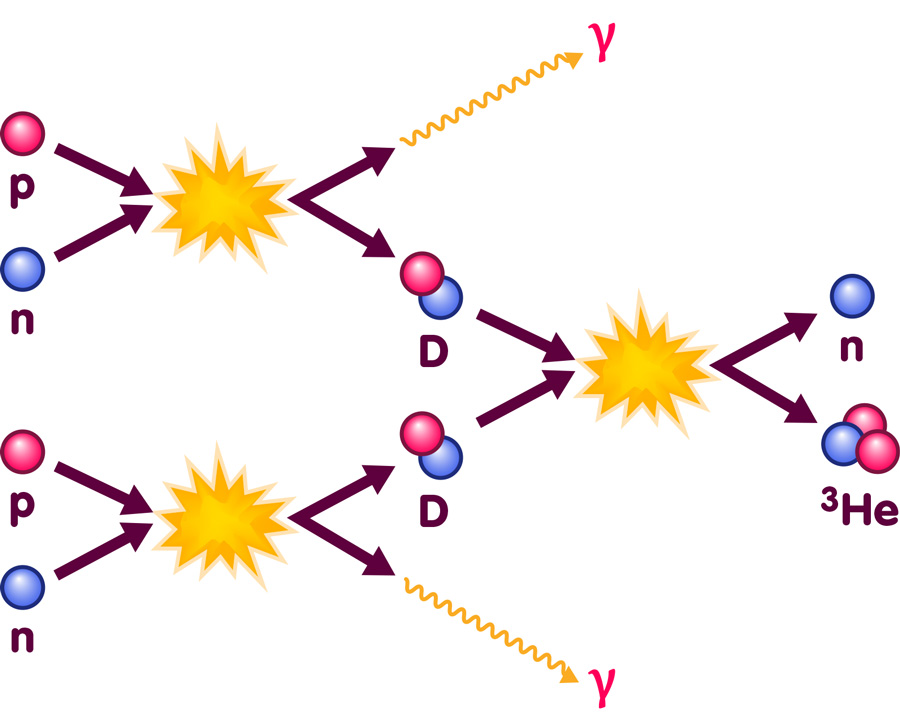
The image illustrates two of the nuclear reactions occuring during Big Bang Nucleosynthesis: It shows protons and neutrons combining to form deuterium nuclei (D, containing one proton and one neutron), accompanied by the emission of high energy photons (denoted as γ); furthermore, it shows two deuterium nuclei fusing to produce one nucleus of helium-3 (with two protons and one neutrons) and one free neutron.
Taking into account a wealth of nuclear reactions similar to the ones pictured above, one can then apply general statistical formula which govern the relative abundances of the different matter constituents. What nuclei are produced, and in what amounts, is the result of a race between the various nuclear reactions on the one hand and the inevitable cooling that accompanies the expansion of the universe on the other. (More details about the physics behind Big Bang Nucleosynthesis can be found in the spotlight text Equilibrium and Change.)
As it turns out, Big Bang Nucleosynthesis strongly favours the very light elements like hydrogen and helium – not only standard hydrogen (one proton) and helium-4 (two neutrons and two protons), but also the isotopes deuterium (one proton, one neutron), tritium (one proton, two neutrons) and helium-3 (two protons, one neutron). By mass, about a quarter of the nuclei in the universe should be helium-4. Deuterium, tritium, helium-3 and lithium-7 nuclei should occur in much smaller, but still measurable quantities.
Theory and observation
Big Bang Nucleosynthesis was incapable to produce heavier atomic nuclei such as those necessary to build human bodies or a planet like the earth. Instead, those nuclei were formed in the interior of stars. By the same token, the element abundances we see around us are not the “primordial abundances” right after Big Bang Nucleosynthesis, but have been altered by later stellar processing.
While, in observing far-away objects, we always look back in time, it is impossible to look back directly to the time of Big Bang Nucleosynthesis since until a much later cosmic time of 400,000 years, the early universe was completely opaque. Instead, astronomers need to look for objects in the universe in which, to the best of current knowledge, the abundances of various elements are as close to their primordial value as possible, or which allow extrapolation to the primordial value. The nature of these estimates is described in the spotlight text Elements of the past: Reconstructing the original abundances of light nuclei.
To confront theory and observation, it is customary to plot the predictions against a parameter denoted by the greek letter eta, which is defined as the total number of protons and neutrons in our universe, divided by the number of photons in the cosmic background radiation. This ratio is nearly constant over time, and it is directly related to the density of nuclear building blocks in the early universe – an important ingredient for the Big Bang Nucleosynthesis calculations. An overview of the results is shown in the following diagram (more detailed plots can be found in the spotlight text Elements of the past):
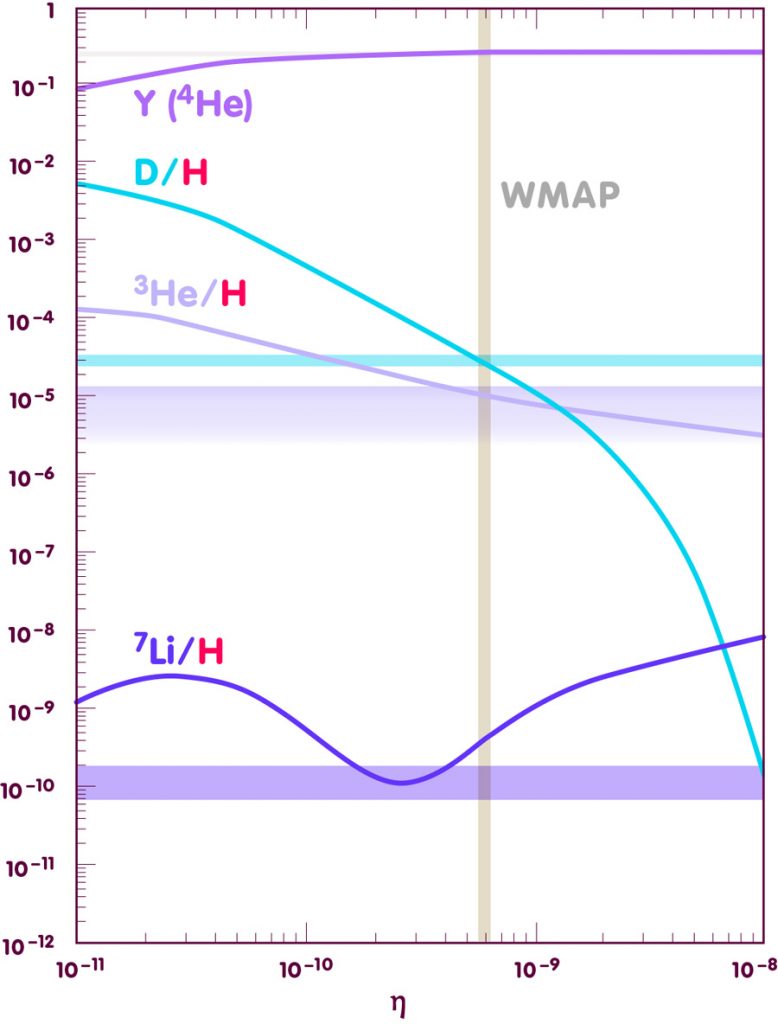
[Adapted from an image by E. Vangioni, Institut d’Astrophysique de Paris]
While the abundance predictions have traditionally been used to fix the correct value for eta, there are different possibilities for measuring that number. Most notably, the presence of particles like protons and neutrons in the early universe leaves a slight, but measurable imprint on the cosmic background radiation. The value of eta that follows from recent high precision measurements with the Wilkinson Microwave Anisotropy Probe (WMAP) is indicated by the vertical beige strip.
As the diagram shows, within all theoretical and observational errors, the Big Bang Nucleosynthesis are impressively accurate. Except in the leftmost part, the pale blue strip indicating the observed value for helium-4 can hardly be distinguished from the theoretical curve. The green deuterium curve meets the pale green strip indicating the observed value almost exactly at the value indicated by the WMAP observations (vertical golden strip), and similarly the WMAP observations, the magenta helium-3 curve and the observed upper limit for helium-3 coincide very well. Only for lithium-7 is there an appreciable gap between prediction and observation though, given the uncertainties of determining the initial abundance of this element from observations, this discrepancy is likely to teach us more about stellar physics than about Big Bang Nucleosynthesis. All in all, this match between theory and observation constitutes one of the big successes of the standard models of cosmology.
Further Information
The relativistic ideas behind this spotlight topic are explained in Elementary Einstein, especially in the chapter Cosmology.
For more information about Big Bang Nucleosynthesis, check out the spotlight texts Equilibrium and Change: The physics behind Big Bang Nucleosynthesis and Elements of the past: Reconstructing the original abundances of light nuclei. Other related Spotlights on relativity can be found in the section Cosmology.
Colophon
Achim Weiss is a scientist at the Max Planck Institute for Astrophysics in Garching near Munich, in Germany. His main area of research is stellar physics.
Citation
Cite this article as:
Achim Weiss, “Big Bang Nucleosynthesis: Cooking up the first light elements” in: Einstein Online Band 02 (2006), 02-1017