Heat that meets the eye
The connection between temperature and the emission of electromagnetic radiation, as well as the consequences for stars, matter disks around black holes, and cosmology
An article by Markus Pössel
Physical bodies (or, speaking more generally, physical systems) that are brought into close contact, close enough for them to exchange energy, evolve towards a state known as thermal equilibrium. In this state, all the systems involved have the same temperature – which is equivalent to saying that the available energy is shared among the systems as evenly as possible. For instance, take two containers, each with a gas consisting of simple molecules (possibly even different species of molecules). Bring the containers’ walls into contact, so heat can flow from one system to the other. In equilibrium, both gases will have the same temperature, and the energy will be distributed evenly among all the different ways the molecules can move: Pick a space direction, and examine the movement of each single molecule in that direction. You will find that, on average, the kinetic energy associated with that particular variety of movement is the same for all molecules in both containers.
There are numerous practical applications for this universal behaviour. We exploit it whenever we switch on a radiator, confident that it will transfer its heat to the surrounding air. We rely on it whenever we switch on a hot plate, confident that the food in the pot we have placed upon it will get hot, as well.
Electromagnetic fields: the system in the background
In all these situations, there is an ever-present additional system, although it is rarely perceived as such: the totality of all electromagnetic radiation filling a certain region of space, from radio waves, infrared radiation and visible light to ultraviolet light and even more highly energetic varieties. In everyday situations, this system is in constant contact with matter. Consequently it, too, evolves towards thermal equilibrium with the surrounding systems.
This has characteristic consequences, namely that hot objects emit significant amounts of thermal radiation and light. The following is a photograph of a hot plate, glowing red:
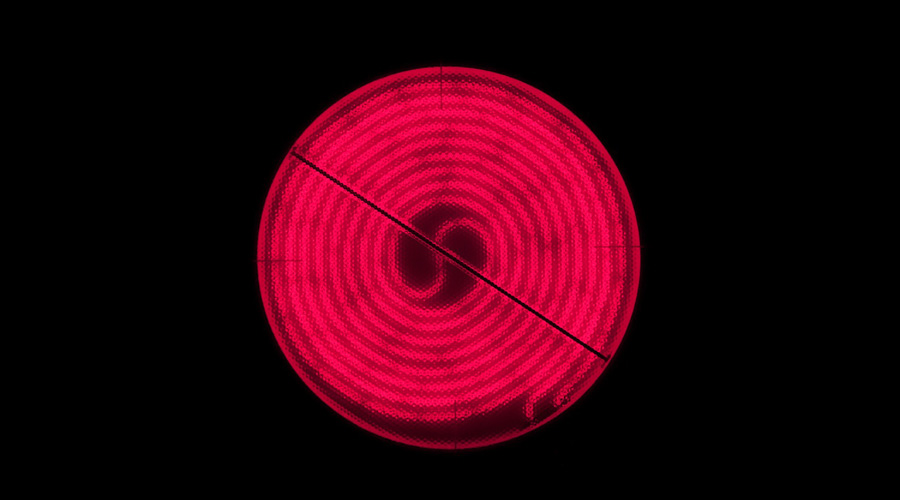
The mechanism behind this phenomenon? On the way to thermal equilibrium, energy spreads evenly throughout all the systems that are in contact. Some of that energy will take the form of electromagnetic radiation – hot bodies will transfer their energy not only to other bodies they are in contact with, but also to the electromagnetic field, in other words: they will emit electromagnetic radiation. The energy transfer – and the energy of the radiation emitted – increases with a body’s temperature.
This type of radiation is called thermal radiation. It has a very typical spectrum – a very typical way in which energy is distributed among the different frequencies; for instance, how much energy is emitted in the form of infrared radiation, and how much in the form of visible light. For thermal radiation, the parameter most important in determining the spectrum is the radiating body’s temperature.
The black body and the laws of radiation
The simplest case is that of an idealized object called a black body, which can absorb and re-emit every kind of electromagnetic radiation, independent of frequency. The spectrum of the radiation emitted by such a body has the shape sketched in the following illustration. What is plotted here is the power of the radiation emitted at different frequencies (in other words, how much energy is emitted at that frequency in a unit interval of time):
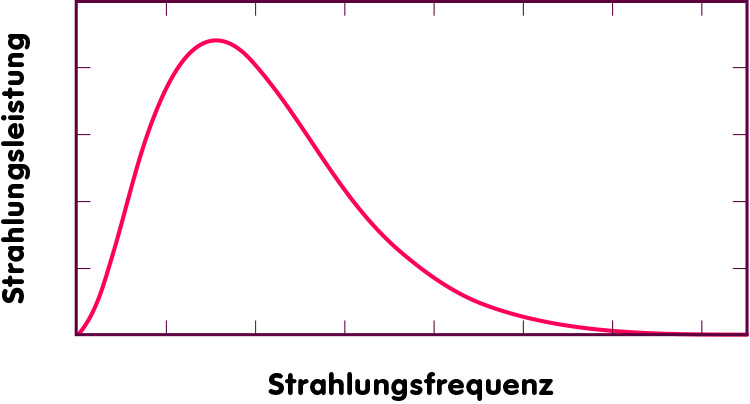
Clearly, a black body emits very little energy at low frequencies (near the left-hand border of the graph). As we proceed to higher frequencies (to the right), more energy is emitted until a maximum is reached at a specific frequency. Further to the right, the emission power falls off. The location of the maximum depends on the temperature – the higher the temperature, the higher the frequency at which the maximal amount of energy is emitted (this is called Wien’s law of displacement). The total amount of energy emitted in the form of thermal radiation is proportional to the fourth power of the temperature: if you double an object’s temperature, it radiates away 16 times more energy! (The law stating this is called the Stefan-Boltzmann law.)
The first to find the mathematical equation for this curve was Max Planck, in the year 1900. Consequently, physicists call this the Planck curve. Incidentally, some peculiar assumptions that Planck made in his attempts to derive the shape of this curve from more fundamental physical laws paved the way for one of the great revolutions in physics – the development of quantum theory.
From everyday objects to stars
Many everyday objects behave quite similarly to black bodies, at least to a good approximation, and in a limited range of frequencies. A hot plate (as in the first illustration) will give a rather dim glow, with most of the radiation emitted at lower frequencies (infrared and red light). As the temperature increases, the colour of hot metal – such as a poker in a fire, or iron in a foundry – goes from dark red to a reddish yellow, accompanied by an increase in brightness. Heated to a temperature of about 4500 degrees Fahrenheit, even a thin metal wire will give off a significant amount of yellowish-white light – which is how ordinary light-bulbs work.
Less close to home, thermal radiation and nearly black bodies are of great importance in astrophysics. To good approximation, the colour and inherent brightness of stars follow the laws of black body radiation. The amount of energy emitted by a star in the form of electromagnetic radiation can be inferred directly from the temperature of certain layers of gas in the star’s atmosphere (called the “effective temperature” of the star) and from the star’s size. Most importantly, the temperature uniquely determines the shape of the black body spectrum. By looking at the spectrum of a far-away star, astronomers can directly deduce its surface temperature!
Just as in the case of the glowing hot plate, the temperature also determines the star’s colour. The following table shows some examples:
| Sirius (α CMa), effective temperature 10200 Kelvin |
| Sonne, effective temperature 5800 Kelvin |
| Capella (α Aur), effective temperature 5500 Kelvin |
| Beteigeuze (α Ori), effective temperature 3100 Kelvin |
[Rendering the colours suitable for display on a computer screen (as RGB colours) is not a trivial task. More about the subtleties involved can be found on Mitchell Charity’s web-page What color are the stars? from which the colour information used in the above table was taken.]
All temperatures are given in Kelvin. Even with the naked eye, one can get a rough impression of these stars’ colours – in the night sky, the “red giant” Betelgeuze (the left shoulder star of Orion the Hunter) indeed glows more reddish than the dog star Sirius.
Black holes and cosmology
While the physics of stars like our sun is a worthy field of study in its own right, what is more interesting in the context of Einstein Online is the role that thermal radiation plays in relativistic astrophysics and cosmology.
When a substantial quantity of matter falls towards a black hole, it can form a swirling maelstrom called an accretion disk. Such disks are extremely hot, and with all that has been said here about thermal radiation, the consequence should not come as a surprise: Accretion disks emit large amounts of energy in the form of electromagnetic radiation. Most of that energy is radiated away at very high frequencies, in the form of X-rays. (More information about this can be found in the spotlight topic Luminous disks: How black holes light up their surroundings, while the corresponding astronomical observations are described in Active black holes: Ultra-hot cosmic beacons).
In cosmology, the ubiquitous cosmic background radiation is an important prediction of the big bang models. It carries a wealth of information about the early universe, and it is, to very good approximation, a black body radiation – the remnant of a time when the universe was filled with extremely hot plasma. At a time of roughly 400,000 years after the big bang, when plasma and radiation had a temperature of 3000 Kelvin, the interactions between the cosmic background radiation and the matter contained in the universe ceased almost completely. Since then, the cosmic background radiation has remained undisturbed, progressively cooling down as the universe expanded further and further. Today, its temperature amounts to a mere 2.7 Kelvin – very close to absolute zero. At this temperature, most of the radiation energy is in the form of microwaves, which is why another name for this remnant of the early universe is “cosmic microwave background radiation”.
Further Information
In the context of Einstein-Online, thermal radiation is especially interesting in connection with black holes and with cosmology. Basic information about these two areas of relativistic physics can be found in the chapters Black holes & Co. and Cosmology of Elementary Einstein.
Colophon
is the managing scientist at Haus der Astronomie, the Center for Astronomy Education and Outreach in Heidelberg, and senior outreach scientist at the Max Planck Institute for Astronomy. He initiated Einstein Online.
Citation
Cite this article as:
Markus Pössel, “Heat that meets the eye” in: Einstein Online Band 04 (2009), 02-1012